

Next: Concluding Remarks
Up: Metallic cohesion
Previous: Electronic Properties
An external electric field  produces an additional
potential
produces an additional
potential  , and a change
, and a change  in the equilibrium electron
density; since
in the equilibrium electron
density; since
 , the kinetic energy (22) does not
change, while the potential energy (23) gives
, the kinetic energy (22) does not
change, while the potential energy (23) gives
 |
(51) |
and
 |
(52) |
the net change in energy is therefore
 |
(53) |
hence one can get the electric polarizability.[1] [2] For a
uniforrmly distributed nuclear charge
[2] For a
uniforrmly distributed nuclear charge  in a sphere of radius
in a sphere of radius  ,
one may use
,
one may use
 and
and
 , where
, where 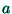 is the average inter-ionic distance; the change
in energy is
is the average inter-ionic distance; the change
in energy is
![$\delta E=-[16/15(aq)^{2}]a^{2}R\left\vert {\bf E}\right\vert ^{2}$](img297.gif) ,
and the polarizability
,
and the polarizability
![$\chi _{e}=\left[ 32/15(aq)^{2}\right] a^{2}R$](img298.gif) ; as
expected it vanishes for large
; as
expected it vanishes for large  .
.
The electrons in a uniform magnetic field  have a
diamagnetic energy
have a
diamagnetic energy
 |
(54) |
where  is the light velocity; hence,
is the light velocity; hence,
 |
(55) |
and, with a uniform distribution of nuclear charges, one obtains[1] [2]
[2] 
 ; the diamagnetic susceptibility is therefore
; the diamagnetic susceptibility is therefore
 .
.


Next: Concluding Remarks
Up: Metallic cohesion
Previous: Electronic Properties
![]() have a
diamagnetic energy
have a
diamagnetic energy